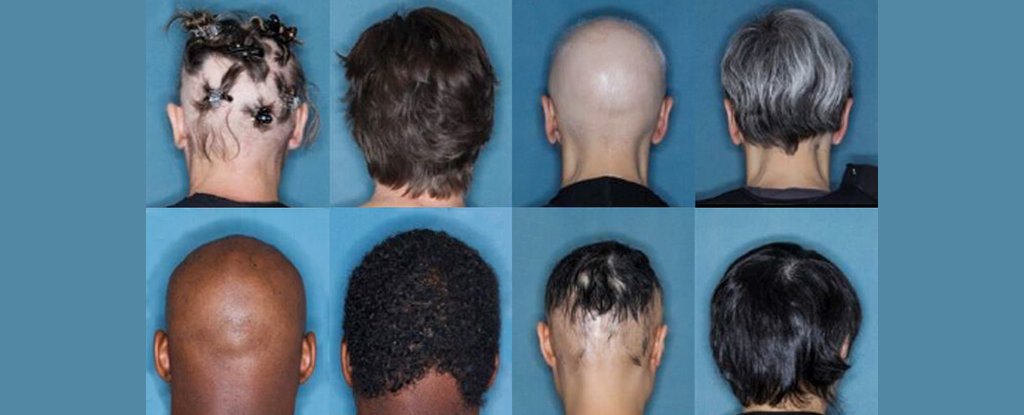
Some of the before-and-after pictures from the trial (Yale)
Rheumatoid joint pain and a serious type of balding called alopecia areata probably won’t seem like they share much for all intents and purpose. One causes joint agony and enlarging, while different prompts sensational, sketchy loss of hair.
Yet, in the two cases, the resistant framework has concluded that the body’s own cells are a danger – in alopecia, this prompts the invulnerable framework going after the hair follicles, while in joint pain, it’s going after tissues in the joints.
Excitingly, notwithstanding, another investigation of a stage three clinical preliminary has shown that the medicines for these two circumstances could likewise be comparable, with a joint pain drug called baricitinib successfully treating alopecia areata in 33% of patients.
This is definitely not a silver projectile for those with alopecia areata, yet a thrilling clinical advancement will ideally before long be accessible for patients as a treatment choice.
“Alopecia areata is an insane excursion, set apart by mayhem, disarray, and significant misery for some, who experience the ill effects of it,” says Yale dermatology specialist Brett King.
“These huge, controlled preliminaries let us know that we can reduce a portion of the experiencing this dreadful infection.”
The explanation this works is a result of a protein called Janus kinase or JAKs. These compounds are essential for a flagging pathway called JAK-STAT, which is associated with a great deal of regions, including the insusceptible framework.
JAK-inhibitors like baricitinib can restrain this invulnerable reaction in certain patients, permitting the hair follicles to start recovering.
The preliminaries were twofold dazed, randomized, fake treatment controlled preliminaries, making them the highest quality level for dissecting how baricitinib functions for those with serious alopecia.
The analysts split 1,200 patients into three gatherings. Members were either given a fake treatment, 2 milligrams of baricitinib, or 4 milligrams of baricitinib for quite a long time. The individuals who were given 4 milligrams of baricitinib had the most emotional outcomes, with more than 33% percent of those patients encountering huge hair development.
The preliminary utilized something many refer to as a Severity of Alopecia Tool (SALT) to have the option to assess the medication’s viability. The score goes from 0 (no balding) to 100 (complete scalp going bald).
Toward the beginning of the preliminary, all members had a SALT score of north of 50, and before the finish of the preliminary, around 35% of the patients on 4 milligrams of baricitinib had a score of 20 or less – an interesting outcome.
Around 20% of patients on 2 milligrams of baricitinib additionally wound up with a score of 20 or less.
“The essential result was a SALT score of 20 or less at week 36. A SALT score of 20 or less has been distinguished as a significant treatment result for patients with serious alopecia areata,” the group writes in their review.
Rheumatoid joint inflammation and an outrageous kind of going bald alluded to as alopecia areata may not appear as though they’ve a ton in continuous. One causes joint throb and enlarging, while the contrary outcomes in emotional, inconsistent absence of hair.
Anyway in each cases, the invulnerable framework has verified that the build’s very own cells are a threat – in alopecia, this outcomes in the resistant framework going after the hair follicles, though in joint pain, it is going after tissues inside the joints.
Excitingly, by and by, a fresh out of the box new examination of a section three clinical preliminary has demonstrated that the solutions for these two circumstances is likewise related, with a joint pain drug alluded to as baricitinib effectively treating alopecia areata in 33% of victims.
This is anything but a silver projectile for these with alopecia areata, but an exhilarating clinical improvement may ideally rapidly be available for victims as a cure plausibility.
“Alopecia areata is a loopy excursion, set apart by bedlam, disarray, and significant despondency for a ton of that experience the ill effects of it,” says Yale dermatology analyst Brett King.
“These monster, oversaw preliminaries illuminate us that we can ease some of the impacted by this awful ailment.”
The reasoning this works is because of a protein alluded to as Janus kinase or JAKs. These proteins are a piece of a flagging pathway alluded to as JAK-STAT, which is worried in an assortment of regions, along with the resistant framework.
JAK-inhibitors like baricitinib are in a situation to restrain this resistant reaction in certain victims, allowing the hair follicles to begin rising once more.
The preliminaries had been twofold dazed, randomized, fake treatment controlled preliminaries, making them the gold standard for dissecting how baricitinib functions for these with outrageous alopecia.
The scientists separate 1,200 victims into three groups. Patrons had been both given a fake treatment, 2 milligrams of baricitinib, or 4 milligrams of baricitinib for a very long time. The people who got given 4 milligrams of baricitinib had presumably the most sensational results, with more than 33% % of these victims encountering crucial hair progress.
The preliminary utilized one thing alluded to as a Severity of Alopecia Instrument (SALT) to can think about the medication’s adequacy. The rating goes from 0 (no balding) to 100 (full scalp balding).
Right off the bat of the preliminary, all individuals had a SALT rating of north of 50, and by the tip of the preliminary, cycle 35 % of the victims on 4 milligrams of baricitinib had a rating of 20 or considerably less – an exhilarating result.
Cycle 20 % of victims on 2 milligrams of baricitinib moreover wound up with a rating of 20 or significantly less.
“The main result was a SALT rating of 20 or considerably less at week 36. A SALT rating of 20 or considerably less has been perceived as a huge cure ramification for victims with outrageous alopecia areata,” the staff composes of their exploration.
“Most victims in whom the main result was met had SALT scores of 10 or considerably less at week 36.”
Unfortunately, this was not liberated from accidental impacts for all victims, with the scientists announcing a spread of signs inside the investigate groups in examination with the controls, along with more regrettable pimples, higher respiratory parcel diseases, complexities, UTIs, and raised degrees of cholesterol.
As well as, because of medication’s usefulness to upset the resistant framework, it might actually furthermore diminish the invulnerable framework’s abilities to shield the build from exact dangers, with raised contaminations ahead of time having been found in these using the medication for joint pain.
With that in contemplations, albeit, a couple of individuals inside the new preliminary exited in light of accidental impacts, proposing that they had been okay aggregate.
Additional investigation is by and by continuous to confirm the security and viability over an extended time, but that is an exhilarating outcome.
The funder for this examination was Eli Lilly and Firm, a drug firm that produces baricitinib underneath the model title Olumiant, by and by endorsed for treating rheumatoid joint pain.
With the section three results from this preliminary currently concluded, and the results needing promising, we may rapidly be seeing this drug remarketed to manage outrageous going bald as actually – ideally offering help for a great deal of victims.
“Most patients in whom the essential result was met had SALT scores of 10 or less at week 36.”
Tragically, this was not liberated from secondary effects for all patients, with the specialists detailing a scope of side effects in the experimental groups contrasted with the controls, including more awful skin break out, upper respiratory parcel diseases, cerebral pains, UTIs, and raised cholesterol levels.
Moreover, because of the medication’s capacity to disturb the invulnerable framework, it can likewise bring down the insusceptible framework’s abilities to protect the body from genuine dangers, with expanded contaminations beforehand having been found in those involving the medication for joint inflammation.
In view of that, however, not very many members in the new preliminary exited because of secondary effects, it were mediocre generally speaking to propose that they.
More exploration is presently progressing to affirm the security and viability over the long haul, however this is an astonishing outcome.
The funder for this exploration was Eli Lilly and Company, a drug organization that makes baricitinib under the brand name Olumiant, at present endorsed for treating rheumatoid joint inflammation.
With the stage three outcomes from this preliminary presently finished, and the outcomes looking encouraging, we could before long be seeing this drug remarketed to regard serious going bald also – ideally giving help to numerous patients.
The examination was distributed in The New England Journal of Medicine.